

On 1 March, 2016, the number of subsidised medicines available to patients in New Zealand In November, 2014, glycopyrronium (Seebri DPI), a LAMA, and indacaterol (Onbrez DPI), a LABA, were added to The range of subsidised medicines used to treat patients with COPD in New Zealand has been transformed over the pastġ8 months.
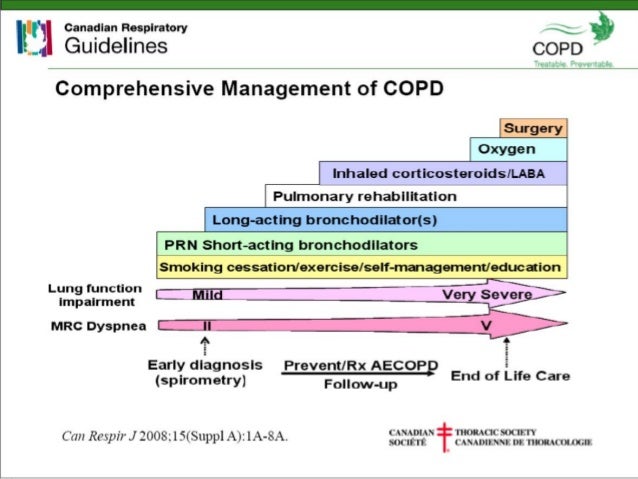
Treatment options for patients with COPD have increased SAMA = Short-acting muscarinic receptor antagonist. **Abbreviations used for inhaled medicines: LAMA = Long-acting muscarinic receptor antagonist, LABA = Long-acting beta 2Īgonist, DPI = Dry powder inhaler, MDI = metered dose inhaler, ICS = Inhaled corticosteroid, SABA = Short-acting beta 2 agonist, The trade names of the various inhaler devices are included in Table 2. An exception has been made in this article,Īs there is the potential for prescriber confusion. *Generally, bpac nz does not use trade names where referring to medicines. Rexair) and budesonide + formoterol (Symbicort, Vannair)

With COPD previous subsidised options required twice daily dosing, i.e.


 0 kommentar(er)
0 kommentar(er)
